The Data Access Process is described in detail in the Atlantic PATH Data & Biosamples Access Policy.
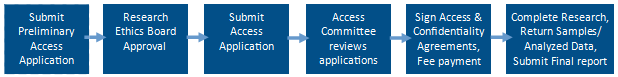
Step 1: Initial consultation:
- We encourage interested researchers to contact us.
- Use the Preliminary Data and Biosamples Application Form and be specific, as the quality of your application will determine the accuracy and timeliness of our assessment.
- Email the completed form to Ellen Sweeney.
- Once we’ve reviewed the application, we will confirm the feasibility and provide a cost-recovery estimate or request more information. Confirmation of feasibility is not a guarantee of data access as final decisions on data access are made by the Atlantic PATH Access Committee
Step 2: Application Process:
- After receiving Research Ethics Board (REB) approval and funding (if applicable), you must submit a Data and Biosamples Access Application Form with supporting documents (i.e proposal, complete REB submission, CV, etc) by email to Ellen Sweeney.
- The Access Committee will review the application and notify you of their decision
Step 3: Approval & Agreements:
- The Approved Researcher and a representative from their associated academic institution must sign a Data Access Agreement with Dalhousie University;
- Terms of payment for the cost-recovery invoice must be agreed upon with initial payment received; then
- The requested de-identified data will be securely transferred to the Approved Researcher.
Step 4: Research:
- During the course of the research project, the Approved Researcher may submit the Access Renewal Form and/or Unanticipated Event/Significant Change Report Form as required.
- In the case of a Privacy Breach, the Approved Researcher must submit a Privacy Breach Report as soon as possible.
Step 5: End of Research:
- Once the data analysis phase has been completed and/or at the time of publication, the Approved Researcher must return derived data to Atlantic PATH, and destroy all original data files provided.
- Once the biological samples analysis phase has been completed, the Approved Researcher must return unused biosamples or derivatives to Atlantic PATH.
Relevant Forms and Documents:
- Data and Biological Sample Access Policy
- Atlantic PATH Preliminary Data and Biosample Access Form
- Atlantic PATH Data and Biosample Access Form
- Atlantic PATH Access Renewal Form Unanticipated Event Significant Changes Form
- Atlantic PATH Reporting a Privacy Breach Form
- Atlantic PATH Data and Biological Samples Return Form
- Atlantic PATH Final Report Form
- Atlantic PATH Publication Policy
For more information about this process, please contact Ellen Sweeney.
Accessing Canadian Partnership for Tomorrow’s Health (CanPath) Data
The Core Questionnaire was completed by all Canadian Partnership for Tomorrow’s Health (CanPath) participants across the country (n=~300,000), including Atlantic PATH participants (n=~34,000). The harmonized data for participants from across Canada is available through the CanPath. Details about the application process are available online and questions can be directed to access@canpath.ca.
